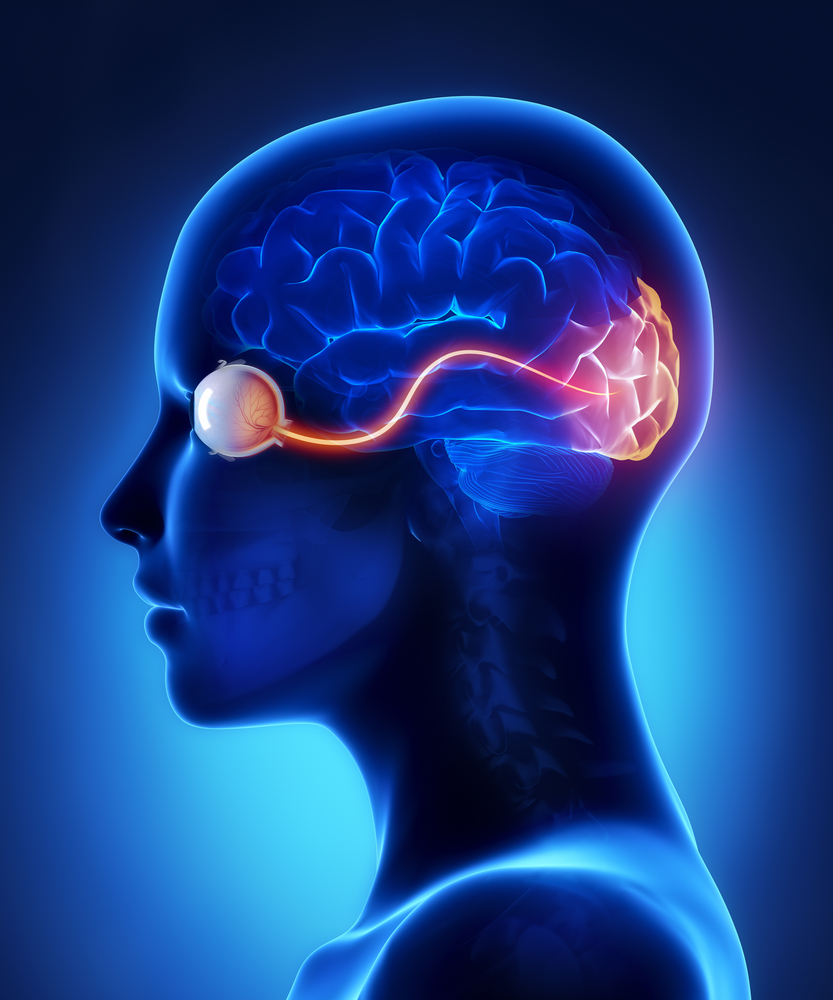
In the retinal tissues of the eye, more than 23 types of RGCs vary significantly in terms of their morphology, connections, and responses to visual stimulation. Those visual transmitting RGCs are the neuronal cells. They all share the defining properties of:
- possessing a cell body (soma) at the inner surface of the retina
- having a long axon that extends into the brain via the optic chiasm and the optic tract
- synapsing with the LGN. The RGCs form multiple functional pathways within the optic nerve to mediate the visual signal
Human beings can see three primary colors: red, green, and blue. This is due to our having three different kinds of color sensitive cone cells: red cones, green cones, and blue cones.
The RGCs connecting to the red and green cones are midget RGCs. They are mainly located at the center of the retina (known as fovea). A single midget RGC communicates with as few as five photoreceptors. They transmit red-green color signals to the parvocellular layer in the LGN (see Figure). The midget-parvocellular pathway responds to color changes, but has little or no response to contrast change. This pathway has center-surround receptive fields, and slow conduction velocities. Because of this pathway, we can see objects precisely in detail and in full color.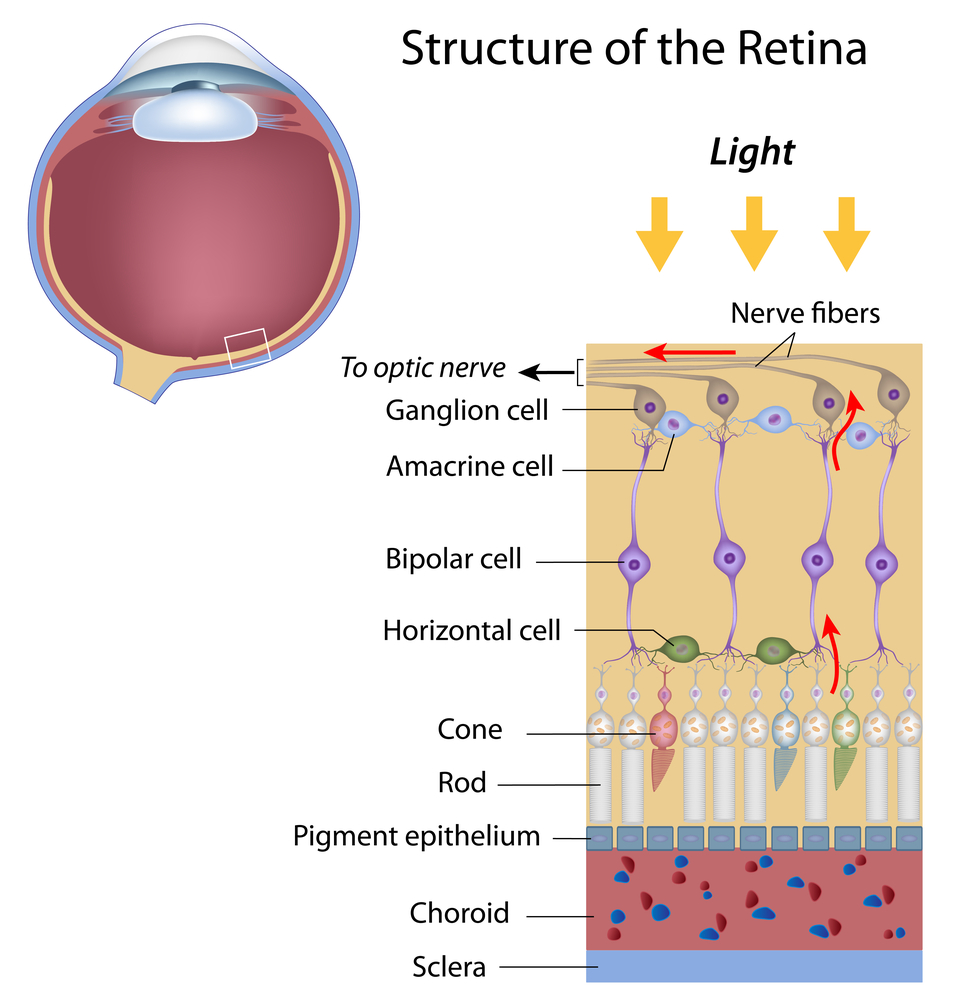
The bistratified RGCs are likely involved in blue color vision. Bistratified cells receive visual information input originally from an intermediate numbers of cones and rods. The bistratified RGCs connect to the koniocellular layers in the LGN (see Figure). The koniocellular neurons form robust layers throughout the visual hemifield and have moderate spatial resolution, moderate conduction velocities, and can respond to moderate-contrast stimuli. They have very large receptive fields that only possess on-center regions (no off-surround regions).
Objects can be seen in the dark with motion and coarse outlines accentuated due to the parasol RGCs. At the periphery of the retina, a single parasol RGC connects to many thousands of photoreceptors (many rods and few cones). The parasol RGCs project their axons to the magnocellular layers of the LGN (see Figure) and are primarily concerned with visual perception. They have fast conduction velocities, can respond to low-contrast stimuli, but are not very sensitive to changes in color.
Finally, humans can see objects in three-dimension courtesy of the crossing over of optic nerve fibers at the optic chiasm. This anatomic structure allows for the human visual cortex to receive the same hemispheric visual field from both eyes (see Figure), thus making it possible for the visual cortex to generate binocular and stereoscopic vision.
Recently, a new type of RGC, called photosensitive RGCs, was discovered. The photosensitive RGCs contribute minimally to our vision, but play a key role in vision regulation. Photosensitive RGCs axons do not have connections to the LGN, but form the retino-hypothalamic tract, and synapse to three other locations in the brain for specific vision regulation functions:
- Pretectal nucleus: involved in reflexive eye movements, thereby helping to target what we want to see
- Midbrain nuclei: involved in controlling the size of the pupil, thus helping to adjust the brightness of objects; and coordinating movement of the eye for focusing
- Suprachiasmatic nucleus: involved in regulating the sleep-wake cycle
A fully functional optic nerve is essential for vision. Obviously, any damage of the optic nerve will sever the precise transmission of visual information between the retina and brain, directly leading to vision distortion and/or vision loss. Damage to the optic nerve can result from:
- Direct/indirect physical damage (e.g. ocular trauma)
- Acute/sub-acute physiological lesion (e.g. infection or inflammation, or malignancy (cancer))
- Chronic neuronal degeneration (e.g. glaucoma, a most common cause of optic nerve damage)
Moreover, the optic nerve is also a very important vivo model for studying central nervous protection and regeneration. At the cell biology level, the RGC axons are covered with myelin produced by oligodendrocytes (rather than Schwann cells of the peripheral nervous system) after exiting the eye on their way to the LGN and thus part of the central nervous system. Scientists have recently acquired more and more evidence that certain types of damage to the optic nerve may be reversible in the future. Therefore, the optic nerve provides a potential window to explore more complicated neuronal degenerative diseases, such as Alzheimer’s disease and Huntington disease.
3/12/15
 Jun Lin, MD, PhD
Jun Lin, MD, PhD
Assistant Professor,
Department of Ophthalmology
New York Eye and Ear Infirmary of Mount Sinai
Icahn School of Medicine at Mount Sinai
—–
 James Tsai, MD, MBA
James Tsai, MD, MBA
Chair, National Eye Health Education Program Glaucoma
Subcommittee President, New York Eye and Ear Infirmary of Mount Sinai Chair
Department of Ophthalmology
Icahn School of Medicine at Mount Sinai


